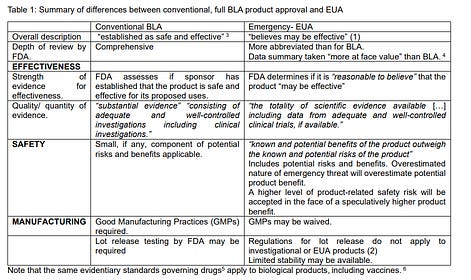
“Safe and effective” is a term defined by law and regulation. However, for an EUA, this term has a different meaning, even though the public has been told otherwise. It only means that a product may be safe and effective, among others.
Under an EUA, each of the standards for “effectiveness, safety, and manufacturing” - and the conventional rigorous proofs and tests to demonstrate these - are thrown out the window. In that case, all that is needed is a “belief” in certain features, trial results - only if available, or any other evidence - that may be based on speculation too.
Sadly, I am not making this up.
All this was exposed by Dr David Wiseman who clearly describes the different standards between conventional, full BLA product approval and EUA (while this is described for the FDA, it seems feasible that many other health regulators globally followed their example).