Abstract
Graphene-based materials (GBMs) have attracted many scientists because of their optical, thermal, mechanical and electronic properties. Their good dispersibility in different type of solvents including water, the possibility to formulate them according to desired function, and the wide surface area, which can allow various chemical modifications, expanded the use of these materials in biological systems. For these reasons, GBMs have been extensively studied in vitro and in vivo in the biomedical field. However, the toxicity and genotoxicity of GBMs must be thoroughly investigated before they can be translated into clinical settings. The main mechanism of graphene toxicity is thought to be caused by reactive oxygen species produced in cells, which in turn interact with various biomolecules including DNA. In this review we aimed to discuss different genotoxicity studies performed with GBMs with specific focus on the different cell types and conditions. By comparing and discussing such reports, scientists will be able to engineer non toxic GBMs for future preclinical and/or clinical studies. In order to allow a safer and faster transition to clinic, future studies should involve state-of-the-art technologies such as systems biology approaches or three-dimensional microfluidic systems, which can better predict the normal physiological scenario.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Carbon, which is one of the basic elements of nature and organic structures, exists in many different forms. The diversity in covalent bonding between the carbon atoms leads to different forms called carbon allotropes. Graphene (G) is a flat monolayer of carbon atoms arranged into a two-dimensional (2D) honeycomb lattice. Its first isolation in 2004 by Novoselov and Geim uncovered the extraordinary features of graphene, which, in the following years, showed a high impact in various industries and research fields including energy, electronics, transport, defence and medicine [1, 2]. In the medicine field, graphene-based materials (GBMs) have attracted many scientists because of their optical, thermal, mechanical and electronic properties [3]. Other properties of GBMs including their good dispersibility in water, their ability to be formulated according to desired function, and the wide surface area, which can allow various chemical modifications, expanded the use of GBMs in living systems. For this reason, GBMs are now used in nanobiotechnology, nanomedicine and in the biomedical field [4, 5].
The unique properties of graphene derivatives such as surface chemistry, electrical conductivity, strong UV absorption, fluorescence quenching ability; makes them one of the most promising materials for imaging, diagnose, biosensor, photodynamic therapy, cancer therapy, regeneration and tissue engineering [6]. Graphene-derived materials can be used as a transducer platform in biosensors [7]. Recommended applications for the family of graphene materials, potential and graphene-based biosensors vary widely [8]. Single nucleotide polymorphisms can be monitored for early identification of genetic disorders such as Alzheimer's and cystic fibrosis [9, 10]. Graphene also exhibits excellent ability as an electron acceptor in fluorescence resonance energy transfer systems [3]. Combining multidimensional structures using GBMs; it allows to increase the optical, electrical and mechanical properties of nanostructures [11].
The inherent self-assembly potential of the nanostructures of the Graphene family has been extensively investigated to form three-dimensional skeletons that may be useful in the biomedical field. Graphene oxide (GO) is versatile due to the presence of functional oxygen groups in its structure; it can form a hydrogen bond with water, which contributes to hydrophilicity. The colloidal properties of the aqueous suspensions of GO may cause the GO layers to self-bond to each other, thus causing the system to gel [5]. In a study, the effect of graphene material on stem cell growth and differentiation was investigated. Cell proliferation of mesenchymal stem cells (MSC) has been shown to be higher on graphene and GO substrates than PDMS substrates; it was found that the degree of mineralization of MSCs cultured on graphene was higher than those cultured on GO [12]. As mentioned above, there are many potential applications of graphene and its derivatives in the biomedical field. However, the toxicity and genotoxicity of GBMs, must be thoroughly investigated before they can be used in healthcare.
The properties of GBMs such as shape, size, load and type of functional groups can affect the interaction of these materials with cells, proteins and other biomolecules. Due to these interactions, the safety and toxicity of GBMs have been extensively evaluated in vitro and in vivo in various studies. In a recent review by Fadeel et al, it has been emphasized that these different properties of GBMs govern their biological effects and scientists should not consider graphene as a single material but rather focus on each individual GBM separately in order to better understand the structure-activity relationships [13].
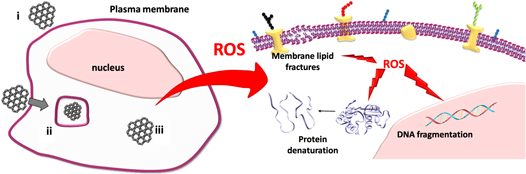
The main mechanism of graphene toxicity is thought to be caused by reactive oxygen species (ROS) produced in cells. The interaction of cells with GO, which is the oxidized form of graphene, causes excessive production of ROS [14], with the increase in ROS production, resulting in oxidative stress [15]. ROS acts as a secondary messenger in many cell signaling cascades. ROS also significantly affects cellular processes and metabolism, such as DNA fragmentation, membrane lipid fractures, and protein denaturation [16]. Protein and DNA damage caused by ROS lead to cell death through apoptotic and necrotic pathways [17, 18]. For example, GO was reported to cause apoptosis and inflammation of lung tissue after inhalation in C57BL/6 mice, whereas minimizing the oxidation state of the material improved biocompatibility [19]. In a study by Li et al, exposure to pristine graphene showed dose-dependent apoptosis and necrosis of murine macrophages [17]. On the other hand, it has been also reported that sheets of GO can be internalized by macrophages through phagocytosis, suggesting that the material could be biodegradable [20]. Following the toxicity studies, the genotoxicity profiles should be also carefully evaluated since any damage to the genetic material may result in the induction or promotion of carcinogenesis, in addition to reproductive impacts if germ cell DNA is compromised. There are two mechanisms that play a key role for genotoxicity induction, namely primary and secondary mechanisms. Either a direct or indirect interaction between the nanomaterial and DNA results in primary mechanisms. In a direct interaction, DNA reactive materials directly contact the genetic material causing physical or chemical damage. In contrast, indirectly acting materials induce genotoxicity through the production of ROS, which in turn damage certain biomolecules including lipids, proteins and DNA (figure 1). In the secondary mechanism, a nanomaterial induces a chronic inflammatory response due to the excessive generation of ROS resulting in the recruitment of immune cells [21]. Therefore it is crucial to understand which mechanisms are involved during GBM-induced genotoxicity.
Figure 1. ROS mediated genotoxicity of GBMs. When cells are exposed to GBMs, the material can (i) make contact with plasma membrane, (ii) be phagocytosed depending on the cell type or (iii) be found free in the cytoplasm. Later, GBMs can result in the production of ROS, which damage certain biomolecules including lipids, proteins and DNA.
Download figure:
Standard image High-resolution imageThere are numerous studies in the literature focusing on the toxicity of GBMs on a variety of cell types. It is important to discuss to compare such reports in order to engineer nontoxic nanomaterials for future preclinical and/or clinical studies. This review focuses especially on the genotoxicity of GBMs, on different cell types/conditions, discussing (i) in vivo studies, (ii) in vitro studies with stem cells, (iii) in vitro studies with cancer cells, and (iv) in vitro studies with somatic cells.
In vivo studies
In vivo models are valuable tools to delineate the systemic effects of nanomaterials and they have been extensively used also in studies involving GBMs. Table 1 summarizes the in vivo studies that involve genotoxicity of GBMs. In a study by Lin et al, a hydroxylated graphene (G–OH) was produced by ball milling as an alternative to GO [22]. Genotoxicity and in vivo biocompatibility of this material were evaluated. For this, Zelanian white rabbits were injected with G–OH at different concentrations by intravitreal administration. The intravitreal injection of G–OH slowly cleared out without causing any damage to cell morphology or eye structure. However, intravitreal injection of G–OH caused several alterations in visual function, such as intraocular pressure, electroretinography and retinal structures. In order to better understand this effect observed in vivo, adult human retinal pigment epithelial cells (ARPE-19) were treated with the material in vitro. It has been observed that when used at a dose of 50 μg ml−1 with a less than 48 h incubation, G–OH caused no cell apoptosis or DNA damage. Cells were observed to go under apoptosis and DNA damage when they were incubated with the same dose of G–OH for more than 72 h. Expression of caspase-3 and p53 was found to be time- and concentration-dependent. However, according to the Comet assay and ROS results, concentration-dependent increase was not observed while time-dependent changes were still applicable. Independently of incubation time, genotoxicity could be observed when the concentration of G–OH was as higher (i.e. 100 μg ml−1). Overall, this study suggested that G–OH did not cause any significant adverse effects on the ocular system, but it had genotoxic effects when used over a long time and at high concentrations [22].
Table 1. In vivo genotoxicity studies with GBMs.
| GBM type | Cell types | Exposure time | GBM concentration | Injection type | Assay | References |
|---|---|---|---|---|---|---|
| G–OH (1.3 nm thickness) | Human retinal pigment epithelial cells | 24, 48, 72 h | 5, 10, 50, 100 μg ml−1 | Intravitreous injection of G–OH | Flow cytometry- apoptosis, Flurimetric-DCFH–DA, Comet assay | [22] |
| GO (0.8 nm thickness) | Spermatozoa | 2 h | 400, 100, 10, 1, 0.1 μg ml−1 | Intravenous injection | ATP/ROS/NO measurements, Comet assay | [23] |
| rGO (1.4 nm thickness) | ||||||
| GO | Bone marrow cells | 7, 28, 56 d | 10, 50, 100, 250, 500 μg kg−1 | Injected intraperitoneally | Chromosomal aberration assayi SCGA assay, SOD/CAT/GSH/MAD levels | [24] |
| GO (1162 nm lateral size) | Bone marrow cells | 5 consecutive days | 10, 20, 40 mg kg−1 | Oral gavage | Micronucleus assay, Comet assay | [25] |
Hashemi et al studied the effect of various GBMs on murine spermatozoa. For this, 8 weeks old Balb/C mice were used. Spermatozoa were obtained from cauda epididymis of mice. Concentration-dependent cyto- and genotoxicity of GO, reduced GO (rGO) (GO reduced using hydrazine), HT-rGO (GO reduced using hydrothermal) and GTP-rGO (GO reduced using green tea polyphenols) layers were investigated on spermatozoa. GO, rGO, HT-rGO layer increased ROS and NO generation, while conjugation of rGO with polyphenols mitigated the effect. For higher concentrations of material, however, all tested graphene nanomaterials showed cytotoxic effects on spermatozoa following 2 h of incubation. The high genotoxicity of the rGO layers was attributed to their capacity to penetrate into the spermatozoa through the ultra-sharp edges of these layers, leading to a specific interaction with the cell nucleus [23].
El-Yamany et al carried out another study to investigate the genotoxic and pulmonary toxic effects of GO nanosheets at different concentrations. For this study, male mature mice were used. These mice were intraperitoneally injected with GO at different doses, and genotoxicity was evaluated at 7, 28 and 56 d after administration. As a result of this evaluation, chromosomal aberrations (structural chromosomal abnormalities) were detected in bone marrow cells of the mice. Furthermore, lung cells showed GO induced DNA fragmentation in a time- and dose-dependent manner. According to these results, GOs accumulated in the lungs and possibly led to DNA damage with oxidative stress in pulmonary tissue [24].
In a study by Mohamed et al the effects of GO nanoparticles on chromosomal and DNA damage were investigated. In this study, GO nanoparticles were administered orally to Male Swiss Webster mice at dose levels of 10, 20, 40 mg kg−1 for one or five consecutive days. Depending on the dose of GO nanoparticle, a significant increase in micronuclei and DNA damage was reported in the study group mice compared to the control group mice. In addition, it has been reported that apoptosis, necrosis, inflammations, and cell degeneration in the liver and brain tissue sections, including histological lesions [25]. Overall these studies indicate that high concentration of GBMs, extended incubation times, dose-depended and ultra-sharp edges cause genotoxicity in vivo.
For the first time in this study, Akhavan et al studied the toxicity of GO nanowalls and reduced graphene nanowalls on bacteria. By measuring the cytoplasmic flow of bacteria, it has been found that the bacterial cell membrane in direct contact with the sharp edges of the nanowalls is damaged and is an effective mechanism for bacterial inactivation. However; their results suggested that direct contact interaction of the bacteria with the very sharp edge of the nanowalls resulted in more damage to the cell membrane of Staphylococcus aureus bacteria as compared to the E. coli ones having additional outer membrane [26].
In vitro studies on stem cells
Due to their mechanical properties, GBMs are good candidates as scaffolds in regenerative medicine. Therefore it is important to assess their toxic and genotoxic effects on stem cells. Table 2 summarizes the studies discussing the effect of GBMs on this type of cells. In a study by Akhavan et al concentration- and time-dependent cyto- and genotoxic effects of single-layer reduced graphene oxide nanoribbons (rGONRs) and rGO sheets on human mesenchymal stem cells (hMSCs) have been investigated. In this study, hMSCs isolated from umbilical cord blood were used. hMSCs were exposed to different concentrations of rGONRs and rGOs. rGONRs showed much higher cytotoxicity than rGOs. DNA fragmentation and chromosomal abnormalities were observed in hMSCs when exposed to rGONRs at a low concentration of 1 μg ml−1 for 1 h. The authors have suggested that the direct contact of the extremely sharp edges of the material was the possible cause of ROS production and DNA damage [27].
Table 2. In vitro genotoxicity studies involving stem cells.
| GBM type | Cell types | Exposure time | GBM concentration | Assay | References |
|---|---|---|---|---|---|
| rGONRs (length of ∼10 μm, 1 nm thickness) | hMSCs | 1 h | 10, 100 μg ml−1 | ROS detection, Comet assay, Giemsa stain | [27] |
| rGOSs (length of ∼2 μm, 1.2 nm thickness) | 96 h | ||||
| rGONPs | hMSCs | 1 h | 1 μg ml−1 | Flurometric DCFH–DA, RNA efflux, Comet assay, chromosomal aberration | [28] |
| rGO | 24 h | 100 μg ml−1 | |||
| GO (3.8 μm) | Spermatogonial stem cells (SSCs) | 24 h | 100 μg ml−1 | ROS detection, Comet assay, membrane integrity | [29] |
| rGO (418 nm) | 24 h | 400 μg ml−1 | |||
| GNO (50–300 nm) | Human adMSCs and bmMSCs | 24 h | 5–300 μg ml−1 | Alamar blue, calcein AM | [30] |
| GONP (20–40 nm) | |||||
| GONR (500–1500nm) | |||||
| 72 h | |||||
Cyto- and genotoxic effects of reduced graphene oxide nanoplatelets (rGONPs) were also investigated by Akhavan et al using hMSCs, isolated from umbilical cord blood. The treatment of hMSCs for 1 h with rGONPs even at a very low concentration (1 μg ml−1), triggered significant cell destruction. On the other hand, when hMSCs were incubated with rGO layers, they exhibited a significant cytotoxic effect only at high concentration (100 μg ml−1). According to the genotoxicity analyses, unlike rGOs, rGONPs were shown to penetrate into the nuclear region of hMSCs causing DNA fragmentation and chromosomal abnormalities even at low concentrations of material [28].
In another study by Hashemi et al, the tyrosine-dependent cytotoxicity and genotoxicity of GO and rGO in spermatogonial stem cells were examined. According to the results, although viability was no affected, high concentrations of GO and rGO significantly increased oxidative stress and DNA damage on these cells following 24 h of exposure, leading to significant toxicity. At lower concentrations of material, no significant genotoxic effects were observed. With this study, it was suggested that spermatogonial stem cells, responsible for the production of germ cells, that transfer genetic material to offspring, are sensitive to GBMs [29].
These studies with stem cells show that direct contact of the sharp edges of GBMs with the cell surface causes ROS production and DNA damage even at low concentrations.
In vitro studies on cancer cells
Thanks to its large surface area and the possibility for surface modifications, GBMs are promising platforms in drug and gene delivery as well as in diagnosis through the coupling of contrast agents. Akhavan et al functionalized reduced graphene oxide nanoribbons with polyethylene glycol (rGONR–PEG) for imaging and phototherapy of human glioblastoma cell line (U87MG). Cytotoxic and genotoxic effects were observed on the cells depending on the rGONR–PEG concentration. When U87MG cells were incubated with 100 μg ml−1 of rGONR–PEG in the dark for 24 h, more than 72% of cell death and more than 29% of DNA fragmentation were observed. At a lower concentration (1 μg ml−1), cell death and DNA fragmentation were reduced to about 11% and 7%, respectively. These results signified that the cyto- and genotoxicity of graphene materials should be carefully studied before combining with the other therapeutic approaches such as photothermal therapy [31].
In a study by Hinzmann et al, the toxicity of pristine graphene, rGO, GO, graphite and ultradispersed detonation diamond (UDD) nanoparticles on human glioblastoma multiforme cells (GBMU87) was evaluated. No changes in morphology of U87 cancer cells were observed after incubating these cells with the materials at 50 μg ml−1 concentration for 24 h. However, the incubation with G and rGO led to a significant decrease in cell viability, while GO, graphite and UDD caused a very small decrease in cell viability. According to the Comet assay results, G, rGO, graphite and UDD caused significant DNA damage, suggesting that there are genotoxic effects on this type of cells [32]. Such studies demonstrate that it is of great importance to test genotoxicity of the materials prior to starting cancer therapy experiments.
In another study, human alveolar adenocarcinoma cells (A549), CaCO2 and Vero cell lines were exposed to different concentrations of GO. After 24 h of incubation, a decrease in viability of A549 cells was observed according to cytotoxicity tests, whereas cell viability was improved in CaCO2 cells. According to Comet assay data, the genotoxicity of micrometer sized (1320 nm) GO flakes was directly concentration dependent. Higher genotoxicity was observed at the highest concentration for the nanometer sized (130 nm) GO flakes. These results also indicated that GO genotoxicity was size-dependent in these cancer lines [14]. These studies have shown that increasing the size and concentration of GBMs causes genotoxic effects on cancer cells. It would be important to evaluate the toxicity of the same material in healthy and tumor cells in order to show its selective anti-cancer activity, if any (table 3).
Table 3. In vitro genotoxicity studies involving cancer cells.
| GBM type | Cell types | Exposure time | GBM concentration | Assay | References |
|---|---|---|---|---|---|
| rGONR–PEG (1 μm) | U87MG | 24 h | 1 μg ml−1 | Comet assay | [31] |
| >100 μg ml−1 | |||||
| G (450 nm–1.5 μm), rGO, GO (100 nm–10 μm), graphite, UDD | GBM U87 | 24 h | 50 μg ml−1 | Comet assay | [32] |
| GO flakes (1320 and 130 nm) | A549, CaCo2 | 24 h | 10, 50, 100 μg ml−1 | Comet assay | [14] |
| GP (450 nm–1.5 μm) | U87, U118 | 24 h | 5, 100 μg ml−1 | Membrane integrity, apoptosis assay | [33] |
| GO, C60, CS (44.9 nm) | MHCC97H , L02 | 24 h | 1, 10, 50 μg ml−1 | Apoptosis assay | [34] |
In vitro studies on healthy primary cells or cell lines
In a study by Chatterjee et al, single-layer graphene oxide (SLGO), few-layer graphene oxide (FLGO), pristine graphene nanoplatelets (GNP), carboxylated graphene nanoplatelets (GNP–COOH) and aminated graphene nanoplatelets (GNP–NH2) were used. Human bronchial epithelial cells (BEAS-2B) were incubated with different concentrations of these materials for 24 h. The genotoxic effects of GNFs were observed not only at DNA damage level but also at the level of DNA repair. According to these findings, GBM-dependent genotoxicity was affecting nucleotide excision repair and non-homologous final assembly repair systems. The alteration and genotoxicity of DNA methylation potentials of GBMs depended on the specificity of GBMs with different physicochemical properties (including number of layer, size and surface functionalization) [35].
Wang et al reported a work on the cytotoxicity and genotoxicity of graphene quantum dots (GQDs) on a fibroblast cell lines (NIH-3T3 cells). NIH-3T3 exposed to GQDs at doses above 50 μg ml−1 did not show any significant cytotoxicity. It has been also shown that GQDs may cause DNA damage in this type of cells. Increased expression of DNA damage-associated proteins (p53, Rad51, and OGG1) was reported. Although GQDs were not in direct contact with DNA and were found to be distributed in the cytoplasm, ROS release caused by GQDs were shown to be responsible for the DNA damage [36].
In a study by Mukherjee et al, neutrophils were shown to mediate biodegradation of GO in a model of human bronchial epithelial cell line (BEAS-2B). GO layers in different lateral dimensions were effectively degraded by neutrophils in this in vitro model. Since the degradation products may have toxicological effects, the effect of degraded GO on BEAS-2B was evaluated. Deteriorated GO did not show any cytotoxicity or DNA damage. With these findings, it was suggested that neutrophils could internalize and degrade GO and this biodegradable GO is not toxic to human lung cells [37].
In this study, Bengtson et al examined the cytotoxicity and genotoxicity of one GO and two reduced graphene oxide (rGO) on the murine lung epithelial cell line FE1. All materials used in the study have been reported to have low endotoxin and inorganic impurity levels. It was also observed that GO produced more ROS than two rGO materials. However, it was observed that the cells exposed to graphene materials for 24 h did not affect cytotoxicity in terms of cell viability and cell proliferation. In addition, no genotoxicity was observed by Comet assay analysis in cells exposed to graphene for 3 and 24 h [38].
Finally, in a study by Wang et al, GO was shown to induce significant cytotoxicity and genotoxicity in human lung fibroblasts (HLF), and it was found to be dependent on the concentration of the material. GO led to mitochondrial dysfunction in the cells and increased the amount of apoptotic cells. At the same time, GO showed genotoxic effects on HLF cells even at the lowest concentration. The surface load quantity and characterization of different GO types played a crucial role on the toxic effect triggered by the material. It has been shown that as the surface load of GO increases, the toxic effect of GO on cells was reduced [39]. In these studies on somatic cells GBMs also showed genotoxicity according to different physicochemical properties and when used at high concentrations. It is important to mention that testing GBMs in various healthy somatic cells is very crucial to show their biocompatibility. Such studies will highlight which tissues are better to test the applicability of GBMs in the biomedical field (table 4).
Table 4. In vitro genotoxicity studies involving healthy primary cells or cell lines.
| GBM type | Cell types | Exposure time | GBM concentration | Assay | References |
|---|---|---|---|---|---|
| SLGO, FLGO, GFNs-pristine, GNP–COOH, GNP–NH2 | BEAS-2B | 24 h | 10, 50 mg l−1 | Comet assay, qRT–PCR, DNA methylation assay | [35] |
| GQDs (lateral size of 40 nm, thickness of 2 nm) | NIH-3T3 | 3 and 24 h | 5, 50 μg ml−1 | IF for XRCC4 and OGG1 expression, flurimetric DCFH–DA assay | [36] |
| GO–S (100 nm), GO–L (10 μm) | BEAS-2B | 0–12 h | 12.5, 25, 50 μg ml−1 | Alkaline Comet assay | [37] |
| LA–PEG–GO (100–200 nm), PEG–GO (50–150 nm) and PEI–GO (200–500 nm) | HLF | 2, 4, 12, 24 h | 1, 10, 50, 100 μg ml−1 | Comet assay, SOD measurement, flurimetric DCFH–DA | [39] |
| GO (2–3 μm) | Murine lung epithelial cell line FE1 | 3 h | 5–200 mg ml−1 | Flurimetric DCFH–DA, Comet assay | [38] |
| rGO (1–2 μm) | 24 h |
Conclusion and future perspectives
There is a continued interest in GBMs and other 2D materials in the fields of biotechnology and biomedicine. Considering the long and costly road towards clinical translation, it is very important to study and evaluate in detail the toxicity and genotoxicity of GBMs. Prior to focus on these biological effects, scientists should carefully characterize the materials [40]. It has been reported that the number of graphene layers, the average lateral size and the carbon-to-oxygen (C/O) atomic ratio play key role in determining the biological effects [13].
There are numerous studies focusing on the genotoxicity of GBMs, as discussed above. These studies will guide researchers to engineer safer nanomaterials with improved properties. It is clear that the evaluation of both toxicity and genotoxicity profiles of GBMs is crucial for a better understanding and prediction of the material behavior in in vivo systems. In this review, we attempted to discuss various genotoxicity studies involving different cell types. We would like to underline that there are not enough reports on the GBMs' genotoxicity effects on immune cells, which are the primary cell types that interact with foreign particles introduced to human body. For example, the immunotoxicological impact of different GBMs on human immune cells, including peripheral blood mononuclear cells, lymphocytes and neutrophils, have been analyzed in different studies [41]. In one of these studies, single-cell mass cytometry and genome-wide transcriptome analyses showed that the presence of amino groups on GO reduced the perturbations caused by GO on immune cell metabolism and increased biocompatibility [42]. Such studies signify the importance of applying systems biology approaches in the field of nanotechnology. With the help of omics approaches, which are analyzing the changes in genes, proteins, metabolites, scientists will have better quantitative and predictive models of nanomaterial behavior in a biological system [13, 43]. For example, in another study by Adamson et al, metabolomic approach was used to better understand the previously reported GNP caused toxicity in cells. Their data suggested that with the help of metabolite profiling approach, the role of CD36 (a phagocytic receptor) was discovered in the cellular response during that GNP-macrophage interactions [44]. Unfortunately, studies in literature applying the systems biology approaches are limited for GBMs and even for other nanomaterials. Therefore, future studies should get use of these technologies to better understand the interactions between the material and the biological system.
Another important point is that, most of the in vitro studies in literature involves 2D culture systems whereas normal human physiological systems involve 3D microenvironment. Use of spheroids, organoids or organ-on-a-chip systems, which are currently used 3D culture models [45], can further help scientists to understand the toxicological and/or genotoxicological effects of GBMs. Polystyrene nanoparticles, for example, have been tested in a multicellular spheroid containing microfluidic chip and results suggested that fluid flow and fluid composition changed the penetration into the spheroid core [46]. In another study by Weiss et al, protein corona formation and composition patterns around nanoparticles were seemed to be changed according to the presence of human blood cells or human serum proteins when tested in a microfluidic system [47]. These studies suggest that 3D-microfluidic systems, which are getting use of both 3D microenvironment and flow properties, are valuable technologies to better understand the spatiotemporal performance of nanomaterials and better guide researchers for a rational design [46, 47]. There are various in-depth review articles which discusses the importance of culture components, co-culture conditions, presence of 3D microenvironment or microfluidic systems during preclinical evaluation of drugs and biotechnological biomolecules [48–51]. Such reports points out that future studies focusing on the toxicity and genotoxicity of GBMs should also consider using physiologically relevant experimental conditions that will mimic an in vivo particle exposure scenario.
Acknowledgments
The authors gratefully acknowledge financial support from Agence Nationale de la Recherche (ANR) (ANR-15-GRFL-0001-05), MIUR JTC Graphene 2015 (G-IMMUNOMICS project), European Union HORIZON 2020 research and innovation programme under MSCA RISE 2016 project Carbo-Immap Grant No. 734381 and under MSCA IF 2017 project IMM-GNRs and through the LabEx project Chemistry of Complex Systems (ANR-10-LABX-0026_CSC).