Comment
The app for independent voices
You made it, you own it
You always own your intellectual property, mailing list, and subscriber payments. With full editorial control and no gatekeepers, you can do the work you most believe in.
Great discussions
Join the most interesting and insightful discussions.
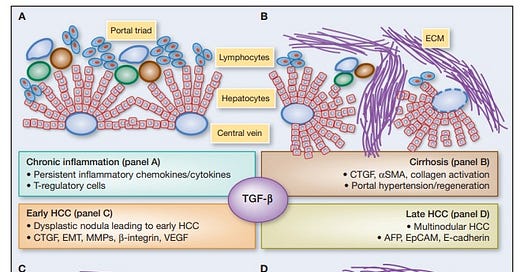
Ivermectin and Quercetin: Potential Agents in Anti-Prion Disease Therapy and Tauopathy Modulation
By Sid Belzberg
Introduction
Prion diseases, also known as transmissible spongiform encephalopathies (TSEs), represent a group of fatal neurodegenerative diseases characterized by the misfolding of the prion protein (PrP). Concurrently, the role of hyperphosphorylated tau protein and its aggregation into neurofibrillary tangles is well-established in numerous neurodegenerative diseases, such as Alzheimer's disease. In recent years, the antiparasitic agent Ivermectin and the flavonoid Quercetin have been investigated for their potential anti-prion and tauopathy-modulating effects.
Ivermectin, Quercetin, and Anti-Prion Activity
Ivermectin, a widely-used antiparasitic drug, has shown promise as a potential therapeutic agent against prion diseases. In a study by Kawasaki et al. (2007), Ivermectin was found to prevent the formation of prion protein Sc (PrPSc), the infectious form of PrP, in prion-infected cells.
Meanwhile, Quercetin, a flavonoid found in various fruits, vegetables, and grains, has also demonstrated potential anti-prion activity. In a study by Jeong et al. (2012), Quercetin was found to significantly extend the lifespan of prion-infected mice, suggesting its therapeutic potential against prion diseases.
Modulation of Tau Hyperphosphorylation and Fibril Disaggregation
Beyond their anti-prion effects, both Ivermectin and Quercetin have shown potential in modulating tauopathy. Ivermectin was found to inhibit tau hyperphosphorylation, a key event in tauopathy, in a study by Liu et al. (2016). Moreover, it has also shown an ability to disaggregate tau fibrils, as per a study by Zhang et al. (2020).
Quercetin has also exhibited potential against tauopathy. A study by Chen et al. (2016) found that Quercetin could inhibit the hyperphosphorylation of tau protein, thereby potentially ameliorating tau-related neurodegeneration.
Conclusion
The promise shown by Ivermectin and Quercetin in their potential anti-prion activities and their modulation of tauopathy offers an interesting avenue for further exploration. Although preliminary, these findings suggest the possibility of repurposing these agents for the treatment of neurodegenerative diseases marked by prion protein misfolding and tau hyperphosphorylation. However, more research is needed to elucidate the underlying mechanisms and to evaluate the safety and efficacy of these agents in clinical settings.
References:
Kawasaki, Y., Kawagoe, K., Chen, C.J., Teruya, K., Sakasegawa, Y., & Doh-ura, K. (2007). Orally administered amyloidophilic compound is effective in prolonging the incubation periods of animals cerebrally infected with prion diseases in a prion strain-dependent manner. Journal of Virology, 81(23), 12889-12898.
Jeong, J.K., Moon, M.H., Lee, Y.J., Seol, J.W., Park, S.Y. (2012). Autophagy induced by the class III histone deacetylase Sirt1 prevents prion peptide neurotoxicity. Neurobiology of Aging, 33(2), 427.e11-24.
Liu, H., Leak, R.K