Comment
The app for independent voices

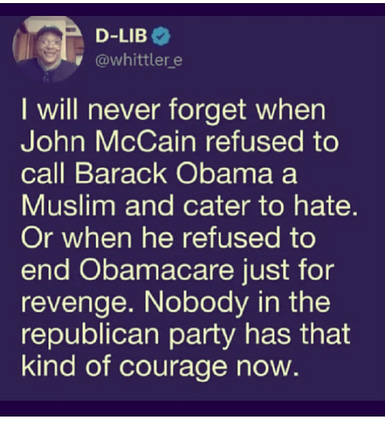
We’re in the DOGE Subcommittee hearing, but where’s Elon? Is the “oversight” in the room with us?
Republicans wanted to talk about NGOs and Stacey Abrams. But, Trump’s out here slapping a for sale sign on the White House lawn—and y’all wanna play games?
Let’s cut the noise and get back to the facts. The people deserve the truth.
You made it, you own it
You always own your intellectual property, mailing list, and subscriber payments. With full editorial control and no gatekeepers, you can do the work you most believe in.
Great discussions
Join the most interesting and insightful discussions.
It absolutely was a bait and switch. And the FDA knew and was a full participant. It's not even arguable. It's right their in their own records.
On Aug 23, 2021, the FDA - to much fanfare - announced it had LICENSED a VACCINE against Covid-19!!! COMIRNATY®, made by the German company BioNTech.
That product's marketing start date for marketing in the United States was Aug. 23, 2021 - it's marketing END date was ALSO Aug 23, 2021. Yes, the same exact day it was licensed for use in the US, it was also pulled from the market and would never be produced.
INSTEAD, the FDA (in its own regulatory letters rto Pfizer and BioNTech) in a now infamous footnote, simply declared that BNT162b2 - the Pfizer product that had ONLY an Emergency Use Authorization - was close enough to be "interchangeable" with the licensed product.
"Interchangeability" is a defined term under the PHSA 42 USC 262(k) and has a whole series of requirements before any two biologics can be declared "interchangeable." The FDA "waived" its "enforcement discretion" and allowed military folks to be forced to take the unlicensed, EUA product - and the entire country fell for it.
My colleagues and I have been screaming about this for 2 straight years now and no one has been listening. For those with a PACER account, I welcome anyone to check these filings.
See Wilson v. Austin, 4:22-cv-00438-ALM, ECF 41.
Or Botello v. US, 1:23-cv-00174-TMD, ECF 13.
The receipts are publicly available and have been. No court will touch this and the DoD, with help from FDA had one of its own officials - Asst SecDef for Health Affairs Terry Adirim - completely unqualified and with no legal authority to do so - simply declared that BNT162b2 were "interchangeable" and therefore that soldiers could be forced to take an EUA product without their consent.