I tend to reference something called The Nylon Problem often in my writing, but the polymer Nylon might not be widely understood except for that it is used as a textile. Nylon was discovered at DuPont by Wallace Carothers and his research team. Wallace Carothers actually discovered quite a few polymers during a time when their existence was not widely accepted by the scientific community, but that is a story for another time. Nylon is a particularly useful polymer that would go on to be used to replace silk in clothing and parachutes, but why is Nylon so useful today and why do we still use it?
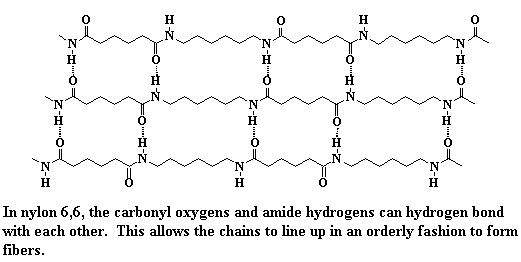
The answer is high strength compared to other similar polymers such as a polyester when we keep the chemical structures as similar as possible. Nylon is a polyamide that is somewhat related to silk while polyesters of a similar carbon structure would be more similar to waxes. The amide functional group enables nylon (and other polyamides) to bond to itself and this is the source of the polymer’s strength. Amides are made from an amine and a carboxylic acid condensing and to form an amide and a water molecule. Silk is the original nylon that nature created from amino acids (molecules that possess amines and carboxylic acids) and enzyme catalysts while nylon was synthesized in a lab at high temperature under vacuum.
Nylons are used quite a bit in automotive and aerospace parts where chemical resistance and strength are needed at a non-cost prohibitive price point because there are other speciality polymers that could do the job of Nylon, but they would cost a lot more. This is an example of The Nylon Problem. Nylon’s properties are so good that substituting it with something else is cost prohibitive so we have been stuck with variations of Nylon since it was discovered in the 1930s.
We utilize nylon in a variety of applications such as synthetic fabrics, monofilaments (finish line), and as injection molded parts for cars. Different nylons or polyamides might yield very different final material properties. Since the initial invetion in the 1930s a whole variety of different nylons situated for different end properties have been synthesized.
Chemists have since been able to create variations of nylon where the amide groups can either be spaced further apart or closer together. We might refer to these polyamides as Nylon 11 or polyamide 11 for placing amides further apart or nylon 4,6 or polyamide 4,6 for amides closer together. Carothers’ original nylon is commonly referred to as nylon 6,6 or polyamide 6,6. The 6 refers to the diacid and the other 6 refers to the diamine. Polyamide 4,6 for instance would utilize succinic acid and hexamethylenediamine. Nylons or polyamides with a single number are made through a different process and utilize only a single monomer (typically in the form of a caprolactam). Polyamide 11 for instance will have better water resistance than polyamide 6,6 but it may also possess less overall strength due to the lack of amide groups.
Amide functional groups are harder to break down compared to polyesters and are not only a source of strength due to interpolymer hydrogen bonding but are also a source of chemical resistance. Because hydrogen bonding is the power behind the strength of nylon it can also be influenced by small molecules like water, which tend to interrupt the hydrogen bonding of the polyamides. Water is like kryptonite to many nylons and can result in materials properties changing and is one reason why it can be very difficult to have things adhere to nylon.
Next time you have some nylon clothing on, stick a sticker on yourself and see how long it stays throughout the day. Do the experiment again when you are wearing cotton. We like nylon for applications where we can reuse it often such as clothing, ropes, fishing line, and automotive parts. Just because the costs of the material might be low does not mean that it is disposable.
Talk to you Friday,
Tony
Subscribe to The Polymerist
A newsletter primarily about how polymer chemistry influences and enables modern life.


