1. Introduction
Of all the human herpesviruses, HHV6 and HHV7 are the most common, infecting nearly every adult, even in developed countries where other herpesviruses are less prevalent. It is thus fortunate that these are also the least virulent herpesviruses. In fact, some people have 100% of their cells infected with HHV6, and even this causes only minor health effects. But how can this happen?
2. Virology
HHV6 and HHV7 are both members of the betaherpesvirus family (of which CMV is also a member). “HHV6” is now known to actually comprise two closely related herpesviruses, named HHV6A and HHV6B. I will discuss HHV6A, 6B, and 7 all together as “HHV6/7”, since these viruses are quite similar.
The overall replication cycle of HHV6/7 is similar to the other betaherpesviruses, but with one important difference. Unusually for herpesviruses, HHV6/7 integrates into host chromosomes (usually near telomeres) during latency (1). If this happens in a germline cell, the result is an offspring where 100% of the cells are latently infected. Inherited HHV6/7 (mostly HHV6) happens in about 0.2–2.9% of the population. Inherited HHV6A in populations with European ancestry can mostly be traced to three separate founder events occurring 23,000 to 105,000 years ago (2). For inherited HHV6B, there were a greater number of founder events, taking place 21,000 to 25,000 years ago.

Although having 100% of your cells infected with HHV6 isn’t great, it’s actually not terrible. One study measured the prevalence of 50 diseases in patients with and without inherited HHV6, and found that the only one with elevated risk was angina pectoris (3). The study also found shorter telomere lengths in patients with inherited HHV6, and the authors speculated that the virus messes with the telomere in which it integrates. All things considered, I would have expected worse effects, considering that 100% of the person’s cells are latently infected. Perhaps similar mechanisms may be at play here to the ones that silence endogenous retroviruses (which also latently infect 100% of your cells).
3. Epidemiology and Transmission
HHV6/7 infect nearly everyone. A 1993 study in the UK found a seroprevalence of 100% for HHV6 in children aged >10 and 96.6% for HHV7 in adults, with high rates at every age group tested (4). A larger cohort study followed 277 American children from birth to age 2, by which time 77% of them were infected with HHV6 (5). In general, HHV6/7 are extremely common.
The primary mode of transmission is likely through shedding in saliva. A study which tracked transmission patterns through sequencing of viral isolates in families found that most infants acquired infections from their mothers (2). Overall, 18 out of 20 sequenced children had the same viral variant as one of their parents.
4. Effects of infection
Primary HHV6B or HHV7 infection, which usually occurs in the first few months of childhood, causes the disease roseola in approximately 20% of cases (6). This disease is characterized by a fever and “rosy” rash. In 10 to 15% of these symptomatic roseola cases (so, ~2% of infections overall), the high temperature of the fever can cause seizures, and roseola is the single largest cause of fever-associated hospital visits in infants. Still, serious complications are rare. In contrast, primary HHV6A infections are not known to cause any disease.
In immunocompromised patients such as transplant recipients, reactivation of HHV6/7 is associated with worse clinical outcomes (7). Even here, though, the direct effects of HHV6/7 are only rarely (in <1% of cases) the cause (8). Instead, the immune reaction to HHV6/7 may precipitate immune rejection of the transplant. Furthermore, HHV6 reactivation can lead to “trans-activation” of latent CMV, another betaherpesvirus but one that’s much worse.
5. A link with Alzheimer’s?
Interestingly, HHV6 and HHV7 levels were found to be elevated in post-mortem brain tissue from Alzheimer’s patients relative to controls (9). There is some evidence for a causal effect based on interaction of these viruses with Alzheimer’s–related gene regulatory networks, but to me, this isn’t a smoking gun. Furthermore, the analysis in that paper was later disputed (10). Their bioinformatic method used to detect HHV6/7 also detected variola virus (smallpox), which is eradicated and certainly shouldn’t be common in brain tissue. The low specificity of their method calls into question their results.
Unfortunately, it’s not possible to say “HHV6/7–negative older adults don’t get Alzheimer’s” since there aren’t enough of those people to study.
In my opinion, immune reaction to herpesviruses (HHV6/7, or also HSV) is plausible as a contributing factor to Alzheimer’s but I’m not fully convinced. (See also discussion here.) In general, it would be great if an infectious agent were the cause of Alzheimer’s. In that case, it would be much easier to prevent and treat.
6. Conclusions
HHV6 and HHV7 are Mostly Harmless. We should be glad, since if they weren’t, almost the entire human population would be in trouble.
However, given the wide prevalence, I think it’s important to understand what exactly is going on here, and it is worth developing treatments/preventions just in case a worse strain emerges.
7. References
1. M. Wood, N. Royle, Chromosomally Integrated Human Herpesvirus 6: Models of Viral Genome Release from the Telomere and Impacts on Human Health. Viruses. 9, 184 (2017).
2. M. L. Wood, C. D. Veal, R. Neumann, N. M. Suárez, J. Nichols, A. J. Parker, D. Martin, S. P. Romaine, V. Codd, N. J. Samani, A. A. Voors, M. Tomaszewski, L. Flamand, A. J. Davison, N. J. Royle, Variation in human herpesvirus 6B telomeric integration, excision, and transmission between tissues and individuals. eLife. 10, e70452 (2021).
3. A. Gravel, I. Dubuc, G. Morissette, R. H. Sedlak, K. R. Jerome, L. Flamand, Inherited chromosomally integrated human herpesvirus 6 as a predisposing risk factor for the development of angina pectoris. Proc Natl Acad Sci USA. 112, 8058–8063 (2015).
4. D. A. Clark, J. M. L. Freeland, P. L. K. Mackie, R. F. Jarrett, D. E. Onions, Prevalence of Antibody to Human Herpesvirus 7 by Age. The Journal of Infectious Diseases. 168, 251–252 (1993).
5. D. M. Zerr, A. S. Meier, S. S. Selke, L. M. Frenkel, M.-L. Huang, A. Wald, M. P. Rhoads, L. Nguy, R. Bornemann, R. A. Morrow, L. Corey, A Population-Based Study of Primary Human Herpesvirus 6 Infection. N Engl J Med. 352, 768–776 (2005).
6. R. C. Stone, G. A. Micali, R. A. Schwartz, Roseola infantum and its causal human herpesviruses. International Journal of Dermatology, 7 (2014).
7. D. M. Zerr, HHV-6 Reactivation and Associated Sequelae after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant, 9 (2012).
8. R. R. Razonable, D. M. Zerr, HHV‐6, HHV‐7 and HHV‐8 in Solid Organ Transplant Recipients. American Journal of Transplantation, 7.
9. B. Readhead, J.-V. Haure-Mirande, C. C. Funk, M. A. Richards, P. Shannon, V. Haroutunian, M. Sano, W. S. Liang, N. D. Beckmann, N. D. Price, E. M. Reiman, E. E. Schadt, M. E. Ehrlich, S. Gandy, J. T. Dudley, Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron. 99, 64-82.e7 (2018).
10. S. D. Chorlton, Reanalysis of Alzheimer’s brain sequencing data reveals absence of purported HHV6A and HHV7. J. Bioinform. Comput. Biol. 18, 2050012 (2020).
Subscribe to De Novo
All things synthetic.




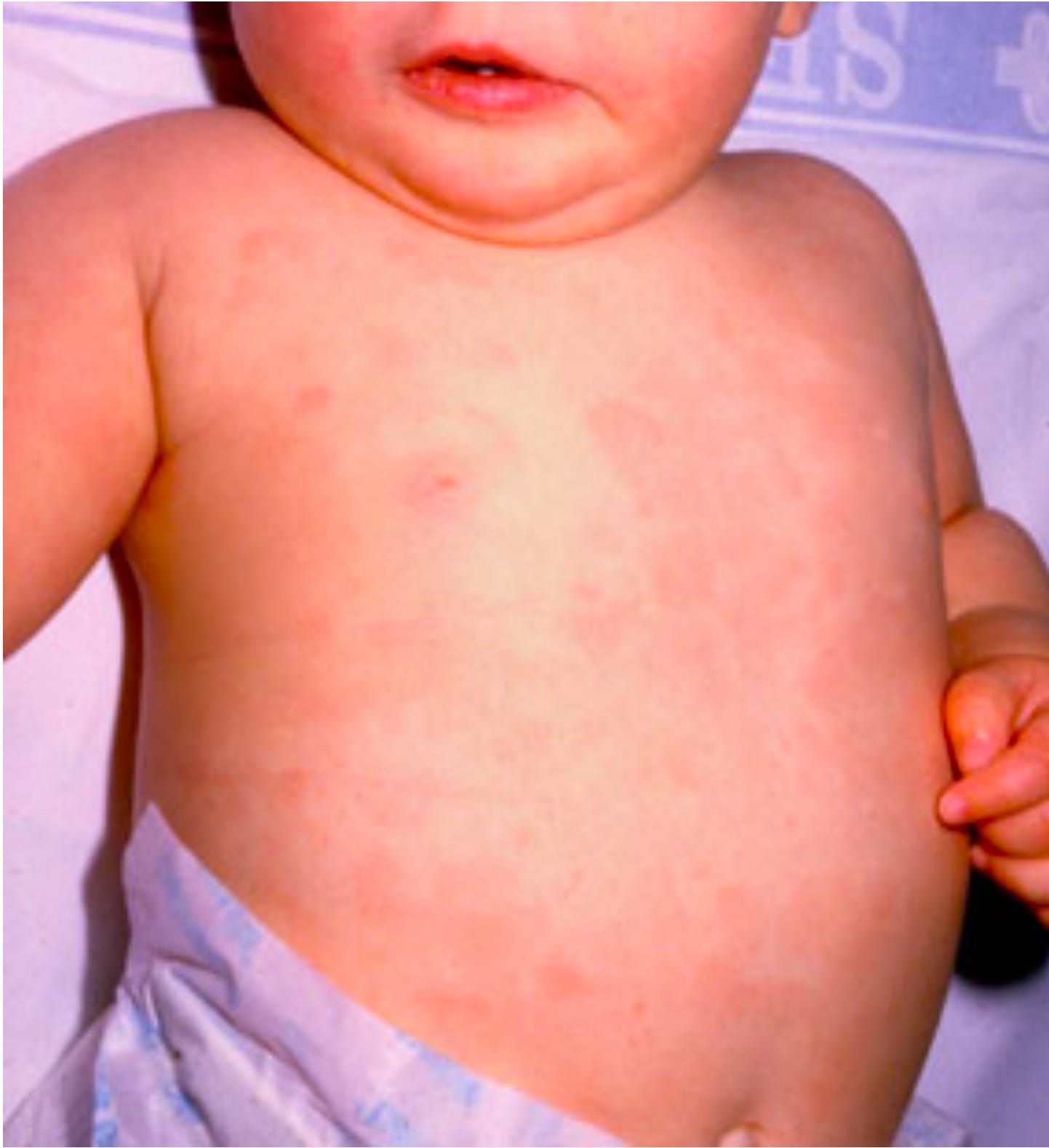
Harmless when alongside a good immune system, being an opportunistic virus, potentially fatal when immunocompromised. My son had disseminated HHV6 for two years post bmt. It led to ulcerative colitis, pancreatitis, infarcted spleen, subdural bleed, seizures, encephalitis and his death.